AspyreRx™ is an FDA authorized, clinically validated treatment for adults 18 years and older with type 2 diabetes
AspyreRx

What is it about?
AspyreRx™ is an FDA authorized, clinically validated treatment for adults 18 years and older with type 2 diabetes. It requires a prescription by your healthcare provider.

App Screenshots






App Store Description
AspyreRx™ is an FDA authorized, clinically validated treatment for adults 18 years and older with type 2 diabetes. It requires a prescription by your healthcare provider.
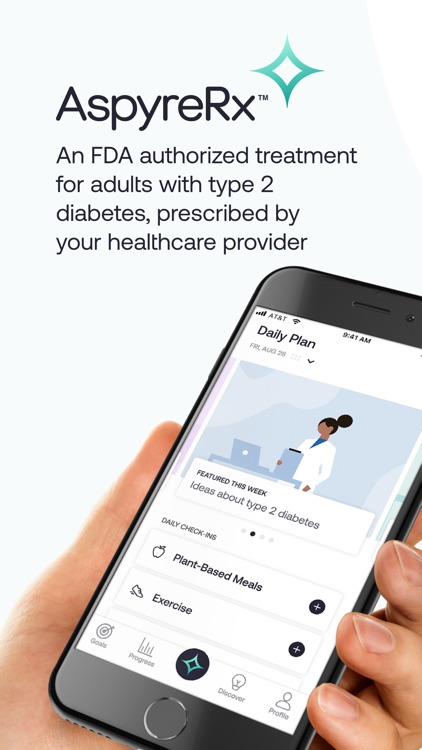
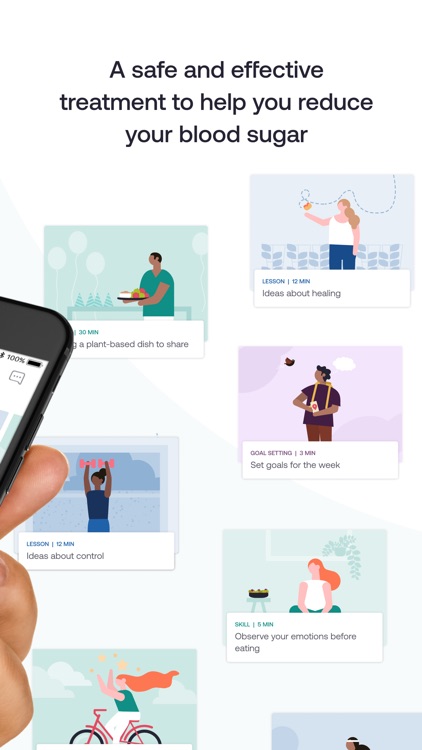
AspyreRx delivers an evidence-based, novel form of Cognitive Behavioral Therapy (CBT) conveniently on your smartphone. Specifically designed for people with type 2 diabetes, AspyreRx combines cutting-edge science with psychology and technology in a practical way to help you understand and change the way you think about and manage your diabetes.
Advance at your pace through interactive therapy lessons, skill-building modules, weekly goal setting and tracking.
AspyreRx helps you:
- Understand the thoughts, beliefs, and emotions that underlie your actions and the choices you make.
- Reframe unhelpful thoughts and reactions, experiment with new ideas, and test them out to see what works for you.
- Reinforce these new mindsets and behavioral patterns through regular practice and guidance, forming lifelong habits.
With AspyreRx, you'll have the support and resources you need to make positive changes to improve your health.
Visit www.aspyrerx.com to learn more and ask your healthcare provider about how AspyreRx could fit into your overall treatment plan.
Clinical study summary:
A 6 month randomized controlled study enrolled 726 participants with type 2 diabetes with a BMI of ≥ 25 kg/m2 and baseline HbA1c between 7 and 11%. Participants who used AspyreRx demonstrated reduced HbA1c (-0.41% at 90 days and -0.30% at 180 days), and had fewer medication increases, compared to participants who did not use AspyreRx.
On average, participants who used AspyreRx also experienced improved fasting blood glucose, reduced systolic blood pressure, reduced weight, improved mood, and improved quality of life scores, compared to participants who did not use AspyreRx.
All participants were treated by their physicians according to standard of care during the study and medication management was continued during AspyreRx usage.
Participants who completed more AspyreRx lessons had, on average, greater HbA1c reduction. For example, those who completed 10 or more lessons in the first 90 days achieved an average 0.4% reduction from baseline at 90 days and an average 0.6% reduction from baseline at 180 days. A statistically significant fewer number of adverse events and serious adverse events were reported among participants who used AspyreRx compared to participants who did not use AspyreRx.
Indications for Use Statement:
AspyreRx is a prescription-only digital therapeutic device intended to provide cognitive behavioral therapy to patients 18 years or older with type 2 diabetes. The device targets behavior to aid in the management of type 2 diabetes in patients who are under the care of a healthcare provider. AspyreRx provides cognitive behavioral therapy as a treatment that should be used adjunctively with standard of care
AppAdvice does not own this application and only provides images and links contained in the iTunes Search API, to help our users find the best apps to download. If you are the developer of this app and would like your information removed, please send a request to takedown@appadvice.com and your information will be removed.