You are using an outdated browser. Please
upgrade your browser to improve your experience.

Clinical Study Launchpad provides an overview of a new clinical study targeting adult patients with severe sickle cell disease
Clinical Study Launchpad
by ARDVRK Technologies, Inc.



What is it about?
Clinical Study Launchpad provides an overview of a new clinical study targeting adult patients with severe sickle cell disease. Using this companion app, prospective patients and family members can more easily learn about the science, treatment protocols, and intended outcomes of this active phase-1 clinical study in a unique and engaging augmented reality form factor.

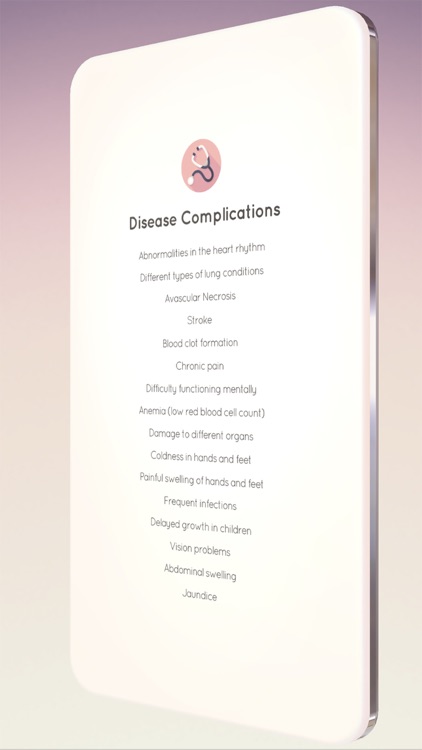
App Screenshots




App Store Description
Clinical Study Launchpad provides an overview of a new clinical study targeting adult patients with severe sickle cell disease. Using this companion app, prospective patients and family members can more easily learn about the science, treatment protocols, and intended outcomes of this active phase-1 clinical study in a unique and engaging augmented reality form factor.
Disclaimer:
AppAdvice does not own this application and only provides images and links contained in the iTunes Search API, to help our users find the best apps to download. If you are the developer of this app and would like your information removed, please send a request to takedown@appadvice.com and your information will be removed.
AppAdvice does not own this application and only provides images and links contained in the iTunes Search API, to help our users find the best apps to download. If you are the developer of this app and would like your information removed, please send a request to takedown@appadvice.com and your information will be removed.