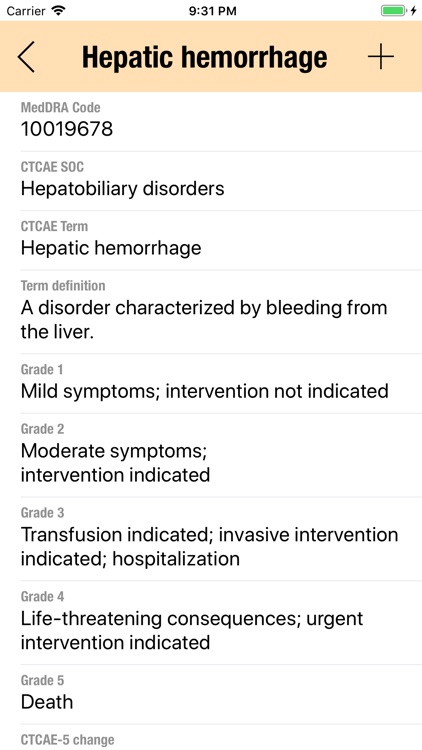
CTC-AE+ is a browsable reference to the CTC-AE list of adverse event (AE) terms commonly encountered in oncology, plus a portable Adverse Event Logger to keep track of all adverse events during a clinical study
CTC-AE+

What is it about?
CTC-AE+ is a browsable reference to the CTC-AE list of adverse event (AE) terms commonly encountered in oncology, plus a portable Adverse Event Logger to keep track of all adverse events during a clinical study.

App Screenshots



App Store Description
CTC-AE+ is a browsable reference to the CTC-AE list of adverse event (AE) terms commonly encountered in oncology, plus a portable Adverse Event Logger to keep track of all adverse events during a clinical study.
The CTC-AE 4 and CTC-AE 5 have been developed from the earlier vocabulary known as CTC (Common Toxicity Criteria). Each AE term is defined and accompanied by a grading scale that indicates the severity of the adverse event. All AE terms are organized by the System Organ Classes (SOCs) defined by the Medical Dictionary for Regulatory Activities (MedDRA).
Adverse events are common phenomena affecting patients being treated for cancer. With the availability of new agents and the multimodality interventions, it is critical to monitor systematically the AEs that are linked to oncology research.
CTCAE is fundamentally intended to be an agreed upon terminology for the designation, reporting, and grading of AEs that occur in oncology research.
########################
CTC-AE serves several purposes
########################
- To standardize AE reporting within the NCI oncology research community, across groups and modalities.
- To facilitate the evaluation of new cancer therapies, treatment modalities, and supportive measures.
- To aid in AE recognition and severity grading.
- To monitor safety data and for regulatory reporting.
- To define oncology research protocol parameters (e.g., eligibility criteria; dose-limiting toxicity; maximum tolerated dose; dose modification).
########################
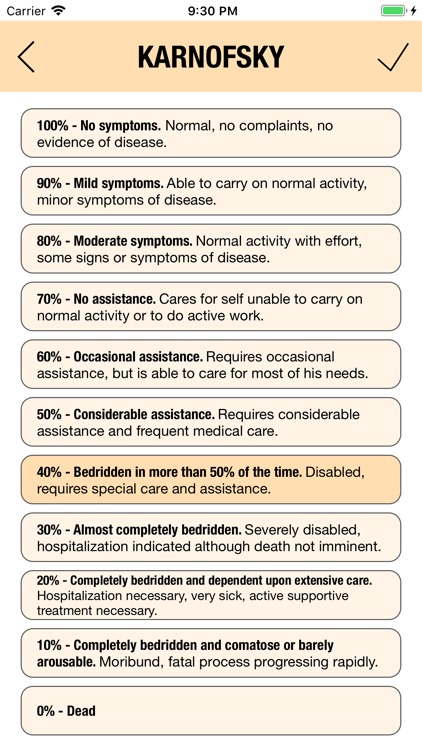
KARNOFSKY and ECOG
########################
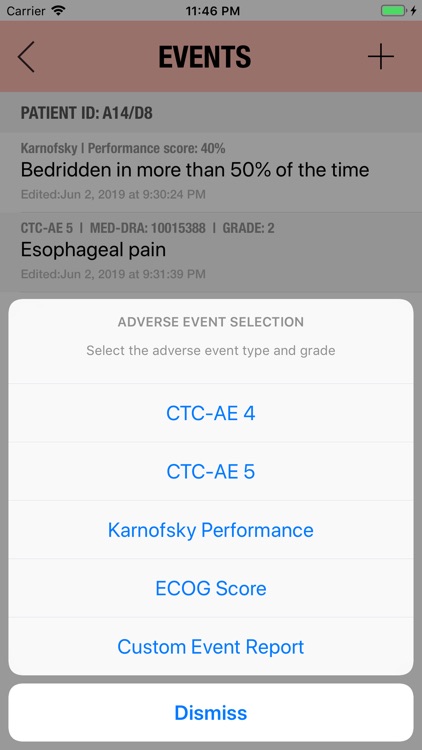
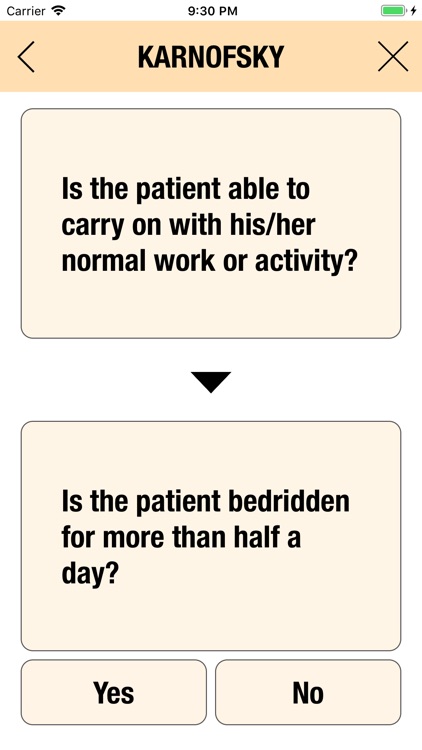
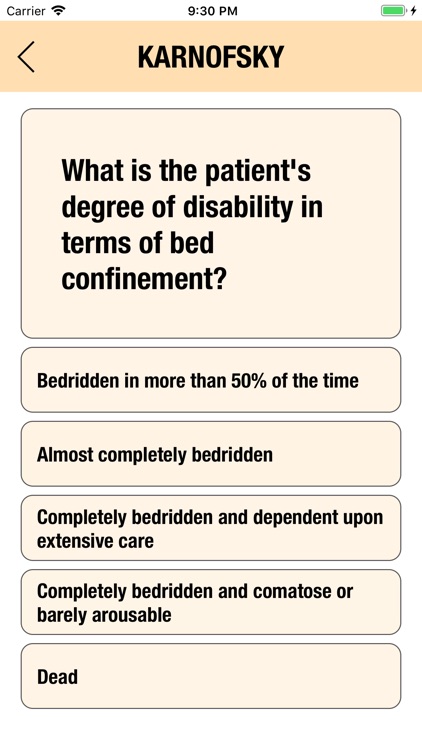
Additionally, CTC-AE+ now includes the two most common performance scores. As a reference and as a guided algorithm when necessary to add the event to the patient's record.
########################
ADVERSE EVENT LOGGER
########################
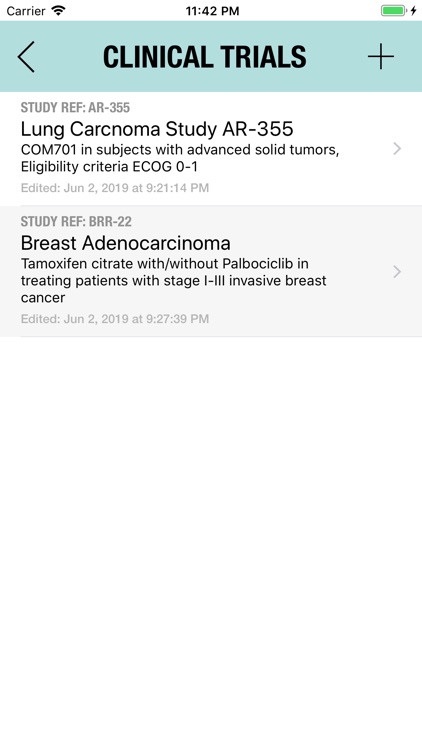
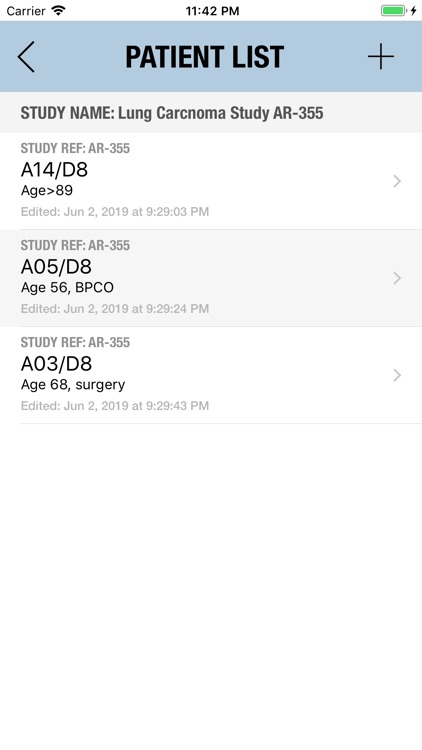
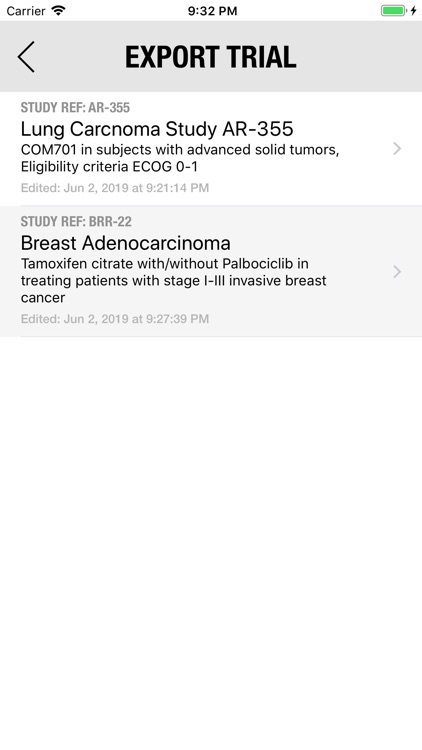
The Adverse Event Logger is a structured local database to help the investigator to keep track of all the adverse events related to the patients participating in a clinical trial.
It is possible to track multiple clinical studies, multiple patients per clinical trial and multiple events per patient. Events can be selected from the CTC-AE 4 and CTC-AE 5 databases, from Karnofsky and ECOG score tables or can be entered as customized events.
For convenience, all data can be exported as an excel CSV file to be imported into other analysis software.
########################
IMPORTANT NOTE
########################
This application is strictly EU GDPR compliant and no clinical study or patient information is saved outside the device or uploaded to any remote cloud resources.
AppAdvice does not own this application and only provides images and links contained in the iTunes Search API, to help our users find the best apps to download. If you are the developer of this app and would like your information removed, please send a request to takedown@appadvice.com and your information will be removed.