Quantum mechanics is a fascinating field that helps us understand the behavior of matter and energy on a microscopic level
Electronify

What is it about?
Quantum mechanics is a fascinating field that helps us understand the behavior of matter and energy on a microscopic level. One of the most important concepts in quantum mechanics is the idea of atomic orbitals.

App Screenshots




App Store Description
Quantum mechanics is a fascinating field that helps us understand the behavior of matter and energy on a microscopic level. One of the most important concepts in quantum mechanics is the idea of atomic orbitals.
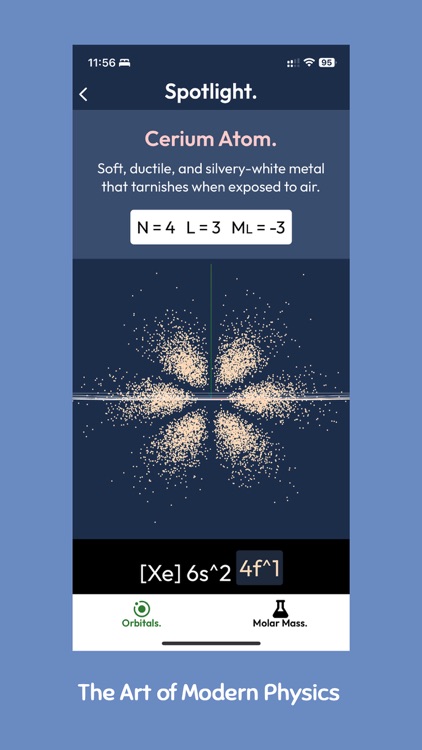
An atomic orbital is a mathematical function that describes the probability of finding an electron in a particular location around an atom's nucleus. Each electron in an atom can be described by a unique set of four quantum numbers, which determine its energy level, angular momentum, magnetic moment, and spin.
The shape of each atomic orbital can be accurately depicted using a formula called spherical harmonics, which creates a visual representation of the electron's probable location around the nucleus. These representations are often shown as a series of dots, each representing a probable location of where an electron might be.
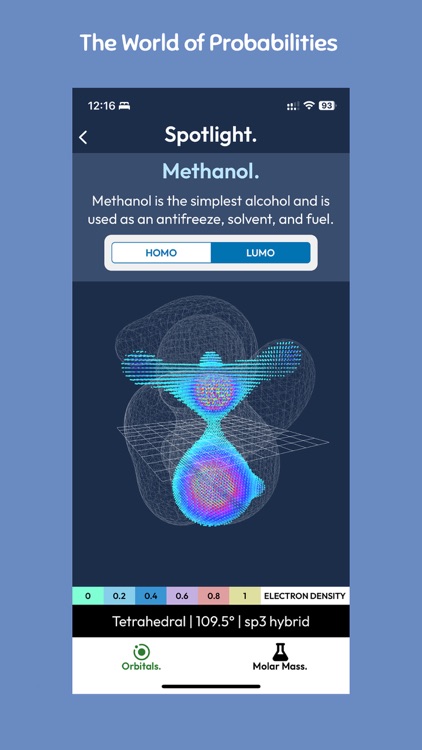
The VSEPR (Valence Shell Electron Pair Repulsion) theory, on the other hand, is a model used to predict the geometry of molecules based on the arrangement of electrons in their valence shells. According to this theory, the electrons in a molecule's valence shell repel each other, and their repulsion determines the molecule's shape.
The VSEPR model predicts a range of molecular shapes, including linear, trigonal planar, tetrahedral, trigonal bipyramidal, and octahedral. These shapes can be used to predict a molecule's physical and chemical properties, such as polarity and reactivity.
This app will provide you these fascinating insights about the nature of how atoms and molecules behave in the real world.
AppAdvice does not own this application and only provides images and links contained in the iTunes Search API, to help our users find the best apps to download. If you are the developer of this app and would like your information removed, please send a request to takedown@appadvice.com and your information will be removed.