
MedWatcher was created in collaboration with the US Food and Drug Administration (FDA) Center for Devices and Radiologic Health (CDRH)

MedWatcher for drugs, vaccines and medical devices

What is it about?
MedWatcher was created in collaboration with the US Food and Drug Administration (FDA) Center for Devices and Radiologic Health (CDRH). Use MedWatcher to get news and official safety alerts for medical devices, as well as drugs and vaccines. MedWatcher is the only app that allows you to report bad side effects or adverse events directly to the FDA to make medical products safer for everyone. MedWatcher is the only app that allows you to report bad side effects or adverse events directly to the FDA to make medical products safer for everyone.

App Screenshots



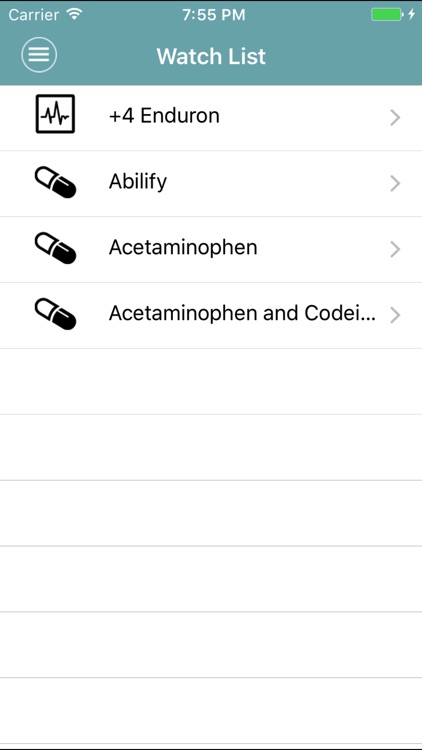
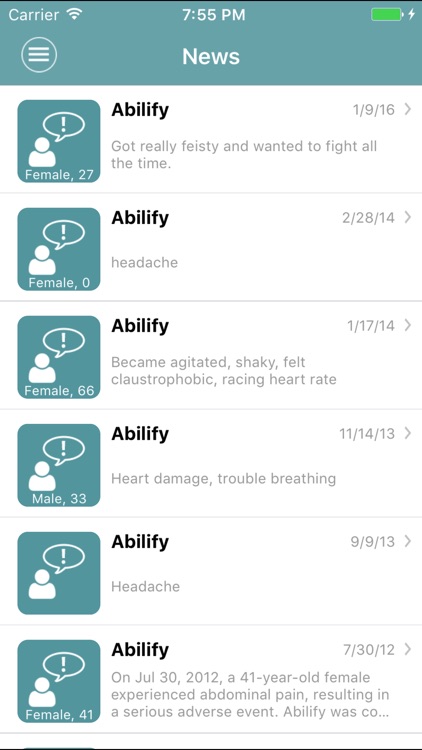
App Store Description
MedWatcher was created in collaboration with the US Food and Drug Administration (FDA) Center for Devices and Radiologic Health (CDRH). Use MedWatcher to get news and official safety alerts for medical devices, as well as drugs and vaccines. MedWatcher is the only app that allows you to report bad side effects or adverse events directly to the FDA to make medical products safer for everyone. MedWatcher is the only app that allows you to report bad side effects or adverse events directly to the FDA to make medical products safer for everyone.
Take control. Participate in your healthcare.
MedWatcher is a mobile tool for both healthcare professionals and the general public. Now with easy-to-read descriptions of a drug’s medical uses and known side effects, and how long it has been on the market. Anyone can submit an adverse event report to FDA using the easy-to-use form on MedWatcher, or post to our online community to talk to others taking the same meds. You can make a quick list of all the drugs, vaccines and medical devices your family or patients use and track the latest developments in the app or via email.
Clinicians will find MedWatcher to be a vast improvement over the paper and fax FDA MedWatch form. No more cramped text boxes or tiny font! Do it at your convenience and fulfill your professional obligations in a convenient way. Get your patients involved in their health.
Actual feedback from people who report bad side effects or adverse events:
“I wanted to be heard.”
“I wanted action to be taken.”
“Reporting an adverse drug reaction can prevent harm to other people.”
(Source PMID: 20658130)
Available as a cross-platform app for tablet and mobile devices. More information at medwatcher.org.
MedWatcher was created by John Brownstein, Nabarun Dasgupta and Clark Freifeld. It is a project of Boston Children’s Hospital, Harvard Medical School, and the University of North Carolina at Chapel Hill. The app was built by Epidemico, a technology company partially owned by Boston Children’s Hospital with exclusive license to HealthMap technology (healthmap.org).
AppAdvice does not own this application and only provides images and links contained in the iTunes Search API, to help our users find the best apps to download. If you are the developer of this app and would like your information removed, please send a request to takedown@appadvice.com and your information will be removed.