
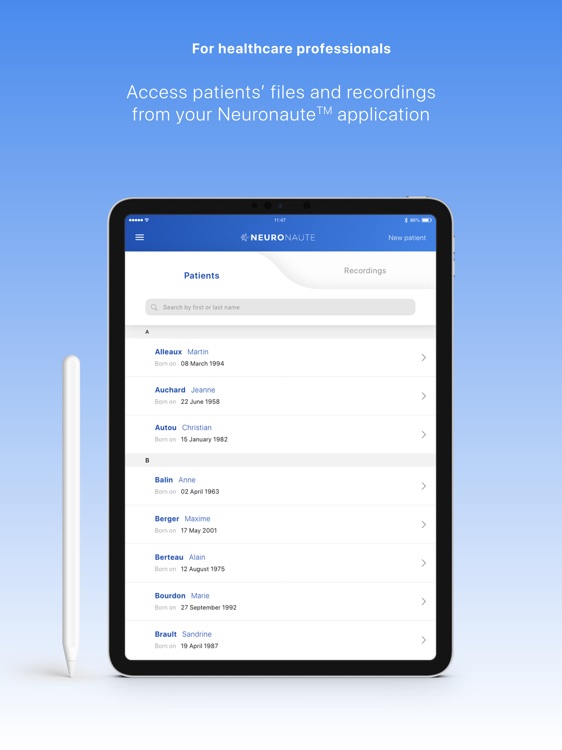
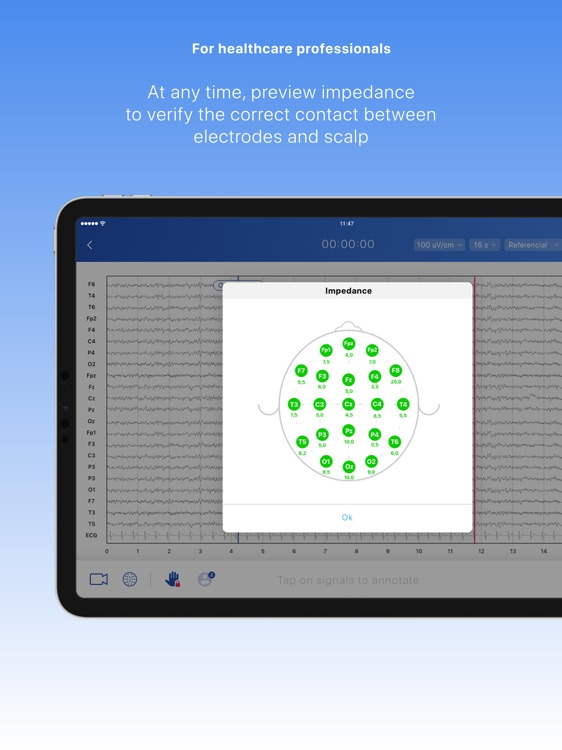
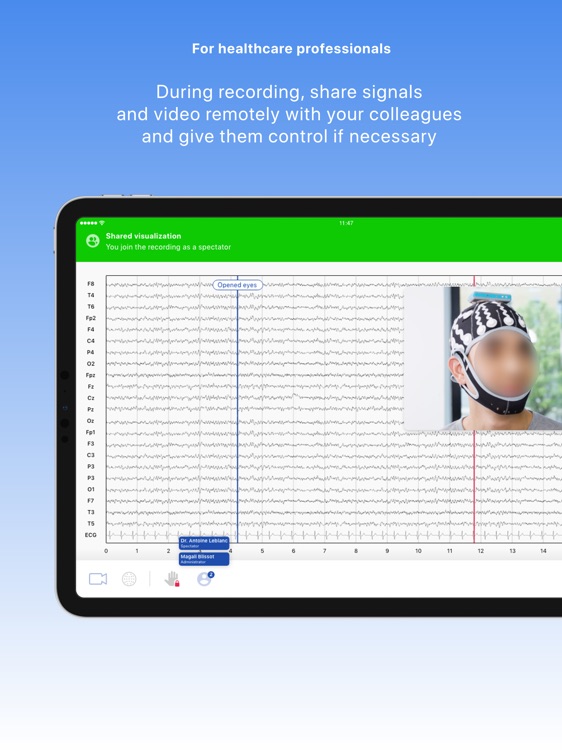
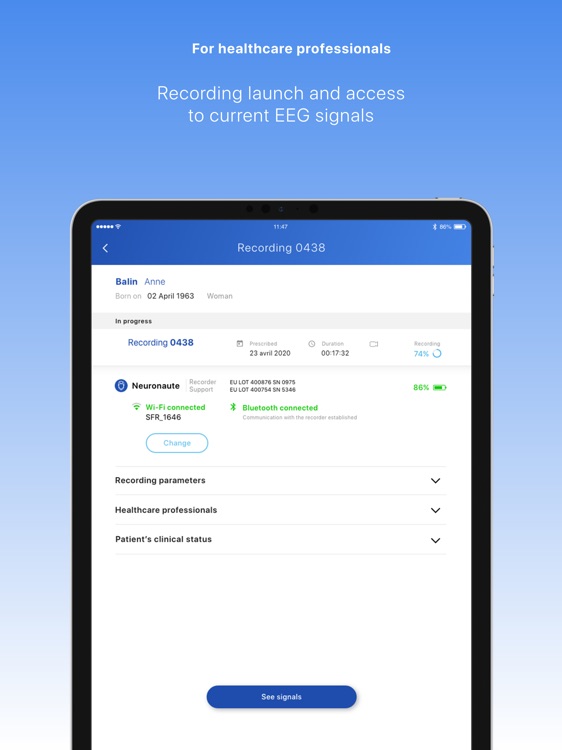
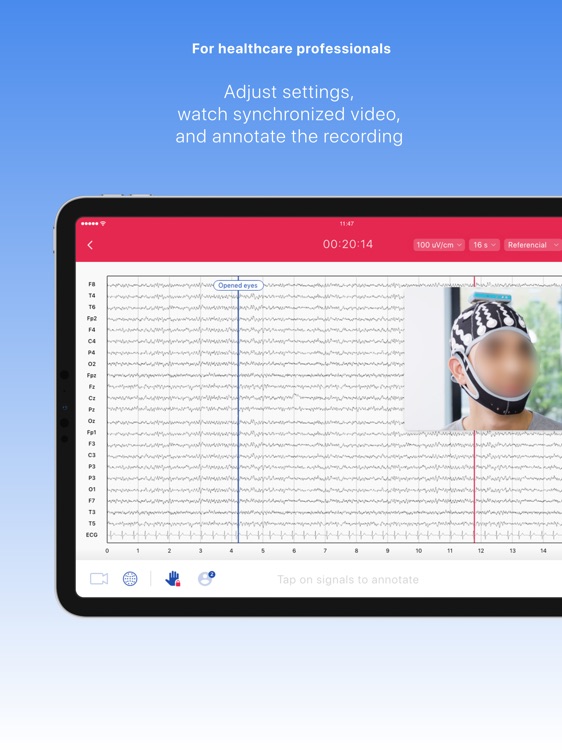
Neuronaute application mobile is a medical device that enables the acquisition, recording, storage, transmission, and display of 21-leads electroencephalogram (EEG) in order to analyze potential neurological disorders

Neuronaute Remote EEG
What is it about?
Neuronaute application mobile is a medical device that enables the acquisition, recording, storage, transmission, and display of 21-leads electroencephalogram (EEG) in order to analyze potential neurological disorders.

App Screenshots



App Store Description
Neuronaute application mobile is a medical device that enables the acquisition, recording, storage, transmission, and display of 21-leads electroencephalogram (EEG) in order to analyze potential neurological disorders.
Neuronaute Application is available:
- In Europe, as a class IIa medical device. It is a regulated health product that bears, under the European regulation of medical devices (93/42 / EEC), the CE 2797 mark.
- In the United States, as a Class I medical device. It is a regulated health product that is, under the United States Medical Device Regulations (21 CFR part 800), exempt from 510 (k) clearance.
Neuronaute application cannot be used alone, it is part of the Neuronaute system and federal law restricts this device to sale by or on the order of a physician.
The application is intended for healthcare professionals.
Users are reminded to:
- Carefully read the instructions supplied with the product before use;
- Consult a healthcare professional if in doubt and before making medical decisions.
More information available at: https://www.bioserenity.com/en/quality-and-regulatory/.
This application is manufactured by BioSerenity, ICM-iPEPS, 47 boulevard de l’hôpital, 75013 Paris - FRANCE.
AppAdvice does not own this application and only provides images and links contained in the iTunes Search API, to help our users find the best apps to download. If you are the developer of this app and would like your information removed, please send a request to takedown@appadvice.com and your information will be removed.