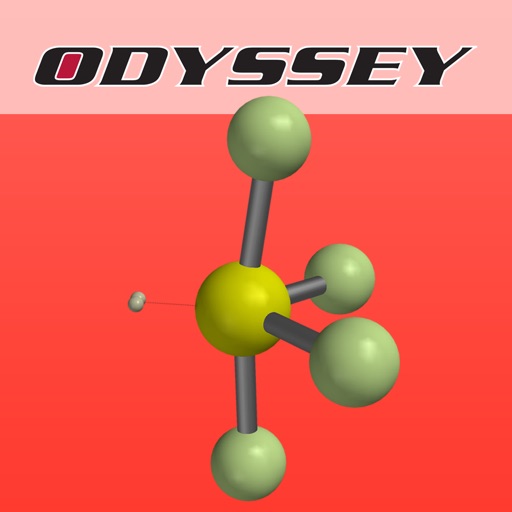
Why are some triatomic molecules linear (carbon dioxide, hydrogen cyanide) and others are bent (water, sulfur dioxide)

ODYSSEY VSEPR Theory
What is it about?
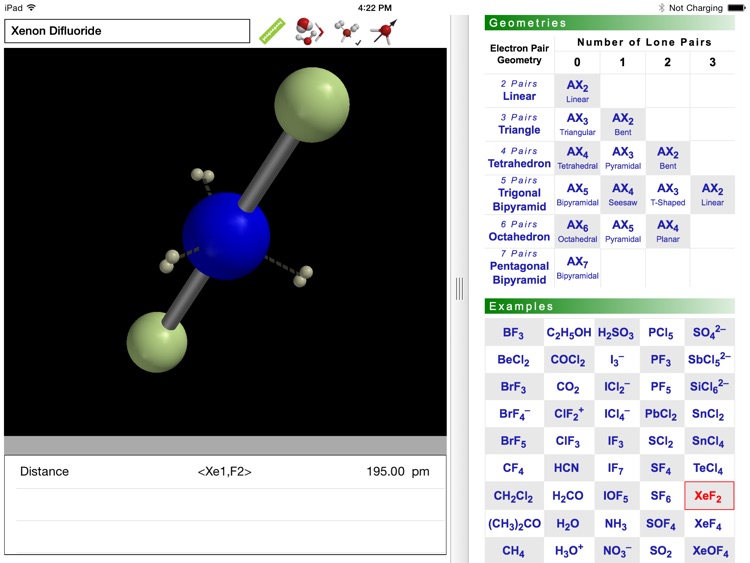
Why are some triatomic molecules linear (carbon dioxide, hydrogen cyanide) and others are bent (water, sulfur dioxide)? And why is the bond angle in water (~105°) smaller than that in sulfur dioxide (~119°)? Valence Shell Electron Pair Repulsion (VSEPR) theory provides surprisingly simple explanations in terms of the electron pairs that surround the central atom of a given molecule. VSEPR theory is described in detail in every introductory chemistry book.

App Screenshots



App Store Description
Why are some triatomic molecules linear (carbon dioxide, hydrogen cyanide) and others are bent (water, sulfur dioxide)? And why is the bond angle in water (~105°) smaller than that in sulfur dioxide (~119°)? Valence Shell Electron Pair Repulsion (VSEPR) theory provides surprisingly simple explanations in terms of the electron pairs that surround the central atom of a given molecule. VSEPR theory is described in detail in every introductory chemistry book.
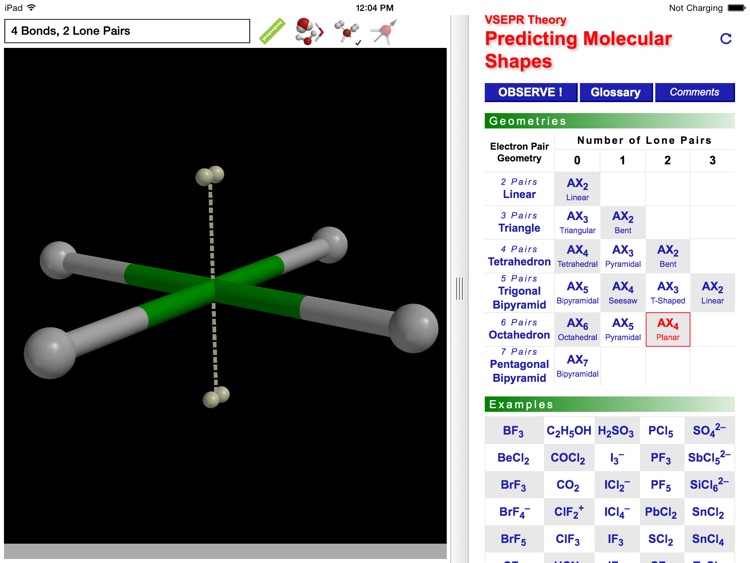
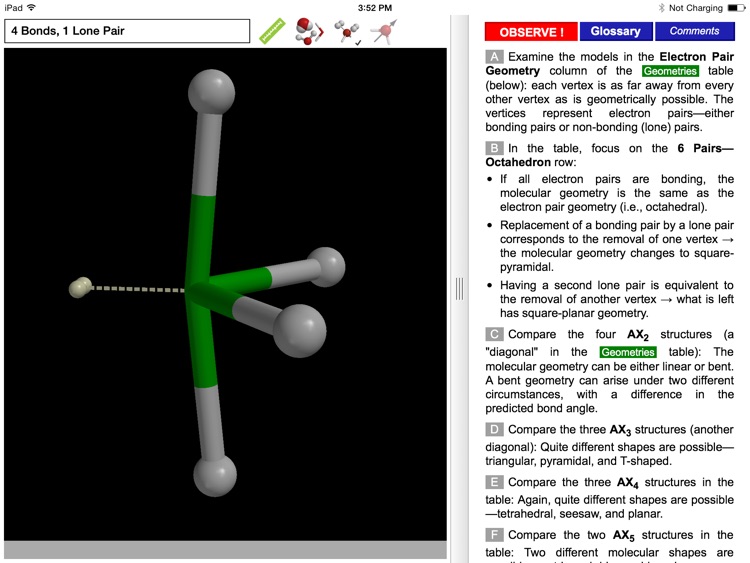
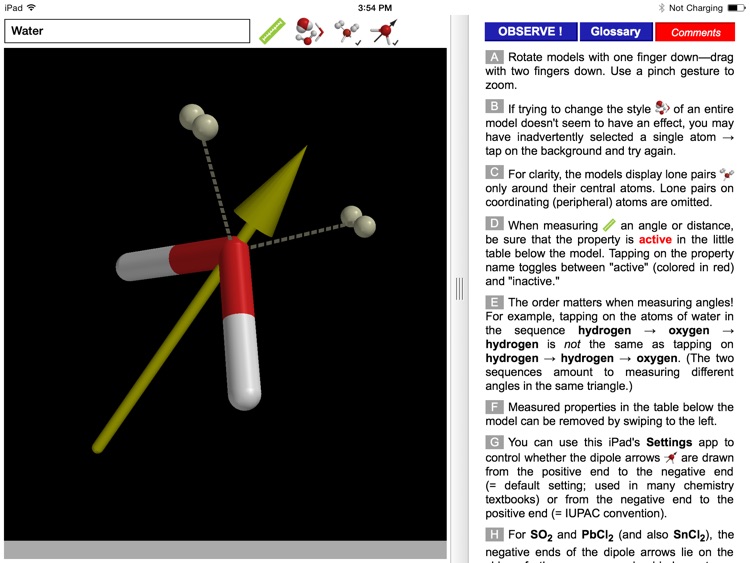
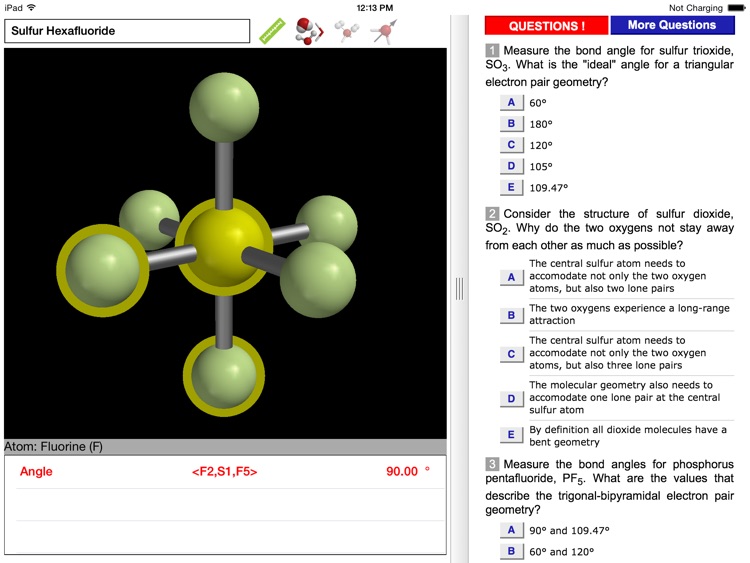
The ODYSSEY VSEPR Theory app provides three-dimensional models of 50 small molecules and ions as well as a set of generic models that illustrate the conceivable geometries of the theory. Each model can be moved and manipulated with simple touch gestures. Bond lengths and bond angles can be queried.
Users can pick from multiple model styles (such as Ball and Spoke or Space Filling), request a schematic display of lone pair positions, and show the molecular dipole arrow. A glossary, comments section, and a set of multiple-choice questions (with randomized options) are also available.
Most learners of chemistry are visually oriented. Complementing the presentation found in any introductory textbook, ODYSSEY VSEPR Theory enables exploration of one of the most fundamental concepts in general chemistry—molecular shape.
AppAdvice does not own this application and only provides images and links contained in the iTunes Search API, to help our users find the best apps to download. If you are the developer of this app and would like your information removed, please send a request to takedown@appadvice.com and your information will be removed.