This app let you prepare solution with your own molarity and concentration
PrepareSolution


What is it about?
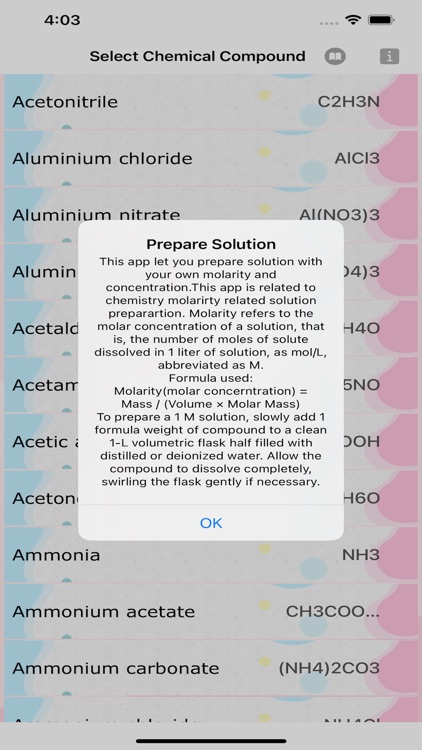
This app let you prepare solution with your own molarity and concentration.This app is related to chemistry molarirty related solution preparartion. Molarity refers to the molar concentration of a solution, that is, the number of moles of solute dissolved in 1 liter of solution, as mol/L, abbreviated as M.\nFormula used:Molarity(molar concerntration) = Mass / (Volume × Molar Mass)\nTo prepare a 1 M solution, slowly add 1 formula weight of compound to a clean 1-L volumetric flask half filled with distilled or deionized water. Allow the compound to dissolve completely, swirling the flask gently if necessary.

App Screenshots




App Store Description
This app let you prepare solution with your own molarity and concentration.This app is related to chemistry molarirty related solution preparartion. Molarity refers to the molar concentration of a solution, that is, the number of moles of solute dissolved in 1 liter of solution, as mol/L, abbreviated as M.\nFormula used:Molarity(molar concerntration) = Mass / (Volume × Molar Mass)\nTo prepare a 1 M solution, slowly add 1 formula weight of compound to a clean 1-L volumetric flask half filled with distilled or deionized water. Allow the compound to dissolve completely, swirling the flask gently if necessary.
In chemistry, the molar mass of a chemical compound is defined as the ratio between the mass and the amount of substance (measured in moles) of any sample of said compound.The molar mass is a bulk, not molecular, property of a substance. The molar mass is an average of many instances of the compound, which often vary in mass due to the presence of isotopes. Most commonly, the molar mass is computed from the standard atomic weights and is thus a terrestrial average and a function of the relative abundance of the isotopes of the constituent atoms on Earth. The molar mass is appropriate for converting between the mass of a substance and the amount of a substance for bulk quantities.
AppAdvice does not own this application and only provides images and links contained in the iTunes Search API, to help our users find the best apps to download. If you are the developer of this app and would like your information removed, please send a request to takedown@appadvice.com and your information will be removed.