Gas Phase Stoichiometry is an ultimate tool for parameterisation of unidirectional chemical reactions that occur in a gas phase
Stoichiometry Gas Phase
What is it about?
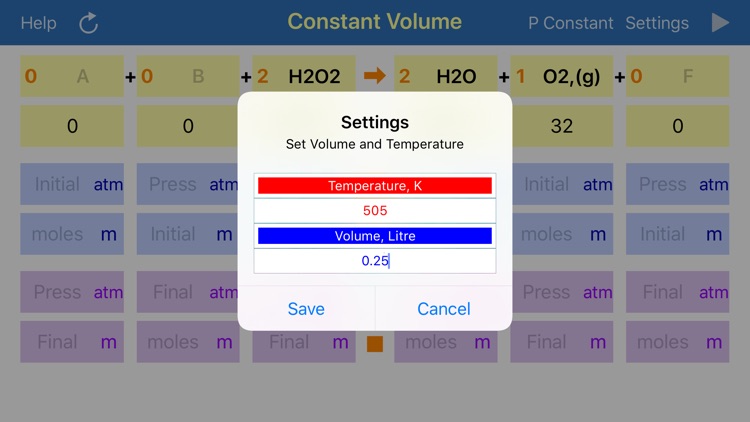
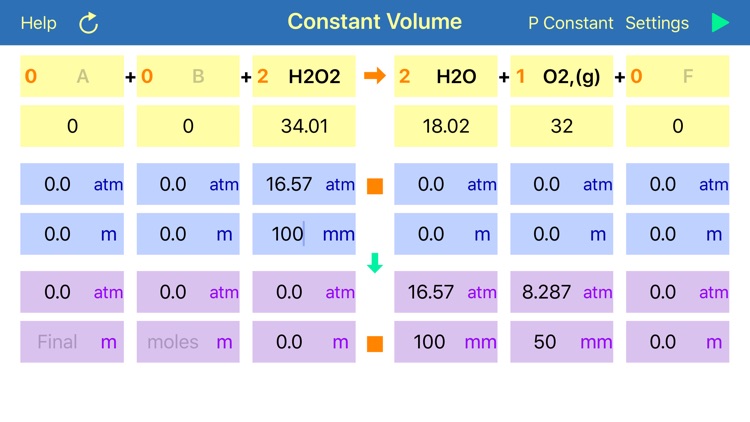
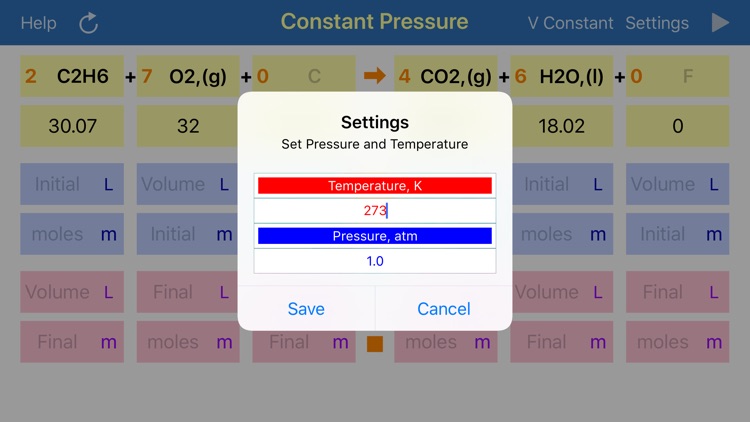
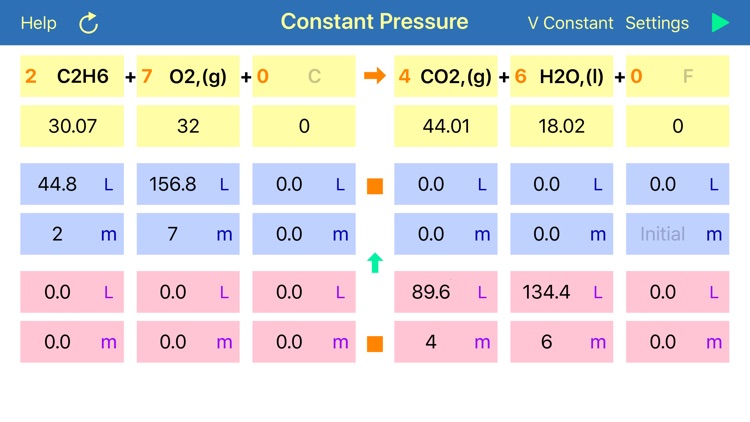
Gas Phase Stoichiometry is an ultimate tool for parameterisation of unidirectional chemical reactions that occur in a gas phase. The application balances chemical reactions, derives molar masses, moles, pressures and volumes of reactants and products based on their chemical formulas, stoichiometric coefficients and user defined temperature, enclosing volume or external pressure. Application provides two layouts: the first is for reactions occurring at the user defined constant volume, where partial pressures of compounds are required for calculation and the second is for reactions occurring at the user defined constant external pressure, where volumes of gaseous compounds are analysed.

App Screenshots



App Store Description
Gas Phase Stoichiometry is an ultimate tool for parameterisation of unidirectional chemical reactions that occur in a gas phase. The application balances chemical reactions, derives molar masses, moles, pressures and volumes of reactants and products based on their chemical formulas, stoichiometric coefficients and user defined temperature, enclosing volume or external pressure. Application provides two layouts: the first is for reactions occurring at the user defined constant volume, where partial pressures of compounds are required for calculation and the second is for reactions occurring at the user defined constant external pressure, where volumes of gaseous compounds are analysed.
User is required to provide initial or final (after reaction accomplishment) pressures (volumes) or moles of any of the reaction component, to calculate all required and obtained pressures (volumes) and moles for all components. If data is available for several components, then limiting reagent will be found based on reaction stoichiometry. Either mole or pressure (volume) should be filled in for each compound, but if both are provided and they do not match, then moles value will have a preference and the stoichiometrically matching pressure (volume) will be recalculated.
For a general unidirectional chemical reaction:
aA + bB -> cC + dD,
where A, B, C, D – are compound formulas and a, b, c, d are stoichiometric coefficients, the components will only react in a defined ratios. Thus, every “a” moles of compound “A” will be needed “b” moles of compound “B”, to produce “c” moles of “C”. If more “B” will be added than it is required, according to the reaction stoichiometry, then all spare amount of “B” will be left unconsumed. In such a case compound “A” will be called a “Limiting Reagent”, as its “small” amount limits or stops reaction before all other reagents are consumed.
While mathematics behind the stoichiometry is rather simple and straightforward, the continuous need to convert moles obtained from balancing chemical reaction to actual pressures or volumes, routinely casts burden upon students and lab workers. In order to facilitate and significantly accelerate the process, the “Stoichiometry Gas Phase” application has been developed.
Please look for working examples at www.volard.wordpress.com.
Important Points:
Either moles or masses can be filled in, but if by mistake both are provided and they don’t match, then the moles value will have a preference.
If one of the compounds is not in a gas phase, then only its mole values should be considered.
Component will be taken into consideration once the stoichiometric coefficient is provided for it.
App uses ideal gas equation to convert moles to pressure or volumes and backwards.
App assigns dot as a decimal point.
AppAdvice does not own this application and only provides images and links contained in the iTunes Search API, to help our users find the best apps to download. If you are the developer of this app and would like your information removed, please send a request to takedown@appadvice.com and your information will be removed.