
Connect your TrueTear™ device with the TrueTear™ app for more insights

truetear

What is it about?
Connect your TrueTear™ device with the TrueTear™ app for more insights.

App Store Description
Connect your TrueTear™ device with the TrueTear™ app for more insights.
Track Your TrueTear™ Device Use
Sync the app with your device to see how often you are using your device. View the Time of the Day and Day of the Week charts to see trends over the last two weeks.
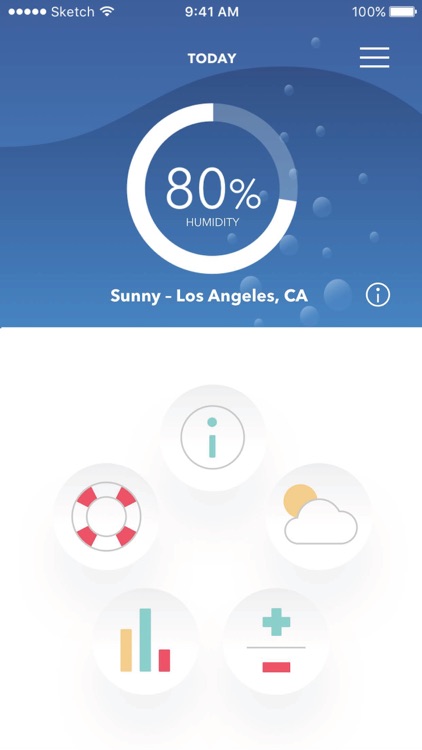
See Real-Time Changes in Weather with Vivid Visuals
View humidity, temperature, wind, pollen, and air pollution conditions in real-time to see how they may impact your dry eyes.
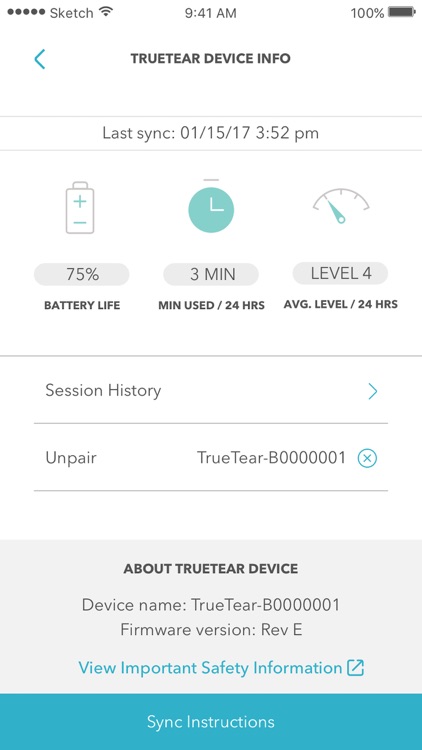
Access the TrueTear™ Device Dashboard
Check your device’s battery life, stimulation level, and session length in a simple chronological list.
Get the Support You Need
TrueTear™ device instructions, helpful hints, and customer service are a tap away.
Note: The TrueTear™ app can be used without the TrueTear™ device.
The TrueTear™ app does not measure, interpret, or make decisions on the data it conveys nor is it intended to provide automated treatment decisions or be used as a substitute for professional judgment. All medical diagnosis and treatment are to be performed under the supervision and oversight of an appropriate healthcare provider. Please follow your doctor’s instructions before using the TrueTear™ device.
INDICATION
TrueTear™ provides a temporary increase in tear production during neurostimulation in adult patients.
IMPORTANT SAFETY INFORMATION
CONTRAINDICATIONS
Do not use TrueTear™ if you have a cardiac pacemaker, implanted or wearable defibrillator, or other implanted metallic or electronic device (eg, cochlear implant) in the head or neck, chronic or recurrent nosebleeds, a bleeding disorder (eg, hemophilia), a condition that can lead to increased bleeding, or a known hypersensitivity (allergy) to the hydrogel material.
WARNINGS
Do not use TrueTear™ around electronic monitoring equipment (eg, heart monitors or electrocardiogram alarms), in the bath/shower, while driving, operating machinery, or during activity in which sneezing/watery eyes may cause risk, areas other than the nose, within 3 feet of shortwave or microwave therapy equipment, around flammable anesthetic mixture (air, oxygen or nitrous oxide). Do not continue using TrueTear™ if your nose is irritated. Safety/effectiveness not established for longer than 6 months or for treating aqueous-deficient dry eye disease. Safety not established in pregnancy, patients under 22 years of age, patients with nasal or sinus surgery (including nasal cautery) or significant trauma, severe nasal airway obstruction or vascularized polyp; active, severe systemic or chronic seasonal allergies; rhinitis or sinusitis requiring treatment; untreated nasal infection; and disabling arthritis, neuropathy, severe dexterity impairment or limited motor coordination.
PRECAUTIONS
Consult your doctor on TrueTear™ instructions before use and on discontinuing use if pain, discomfort or numbness in the nose persists after reducing for higher levels/longer sessions. Remove studs, nose rings, or other nose jewelry before use. Do not use prescription eye medications or nasal sprays 30 minutes before or after using TrueTear™. Consult your doctor before use if you have suspected or diagnosed heart disease. Keep away from children.
ADVERSE EVENTS
Nasal pain, discomfort or burning; short-term electrical discomfort; nosebleed; nasal congestion; headaches; trace blood in nostril; facial pain; sore eye; sinus pain; pain around the eye; runny nose; nasal ulcers; and light-headedness.
Caution: Federal law restricts this device to sale by or on the order of a licensed physician. For the full Directions for Use, please visit https://www.allergan.com/truetear/usa.htm or call 1-800-678-1605. Please call 1-800-433-8871 to report an adverse event.
TRU105492_V2 09/17
AppAdvice does not own this application and only provides images and links contained in the iTunes Search API, to help our users find the best apps to download. If you are the developer of this app and would like your information removed, please send a request to takedown@appadvice.com and your information will be removed.