
You are only able to participate in the Turbu+ programme if enrolled by your Healthcare Professional

Turbu+ Australia

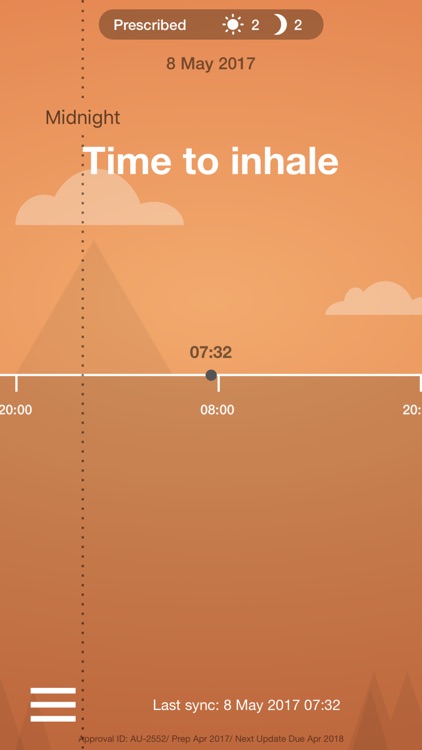

What is it about?
You are only able to participate in the Turbu+ programme if enrolled by your Healthcare Professional.

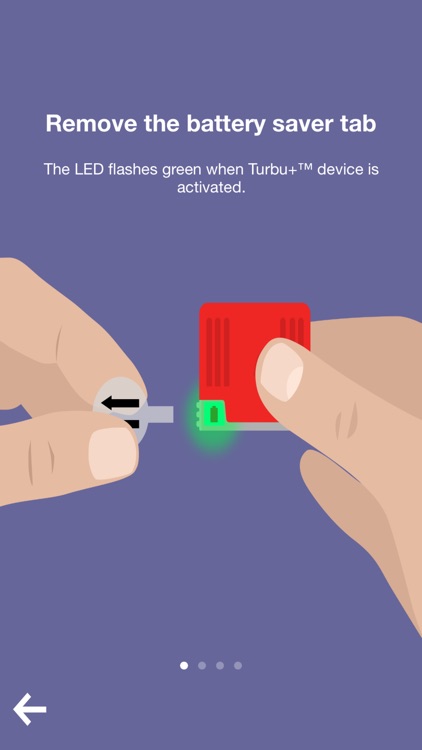
App Screenshots



App Store Description
You are only able to participate in the Turbu+ programme if enrolled by your Healthcare Professional.
Turbu+ is a programme intended to assist people with asthma and/or COPD in recording and monitoring the actuations of prescribed inhaler usage. Users will interact with the programme through a mobile app. This mobile app receives, stores, and displays inhalation/usage events from remote sensors in the compatible Bluetooth-enabled monitoring (Turbu+) device attached to the user’s inhaler, to support them in adhering to their prescribed treatment regime. The app also provides users with pre-configured medication reminders and motivational messages encouraging adherence with their prescribed medication.
The Turbu+ programme is not intended to:
• Diagnose, determine, modify or adapt the healthcare professional's treatment decisions
• Monitor, in real time, the user's medication use
• Determine or change the user's medication or treatment plan or to recommend such changes
• Alter or analyse inhaler usage information, other than by formatting the data for display.
Turbu+ is a patient support programme which has been designed, developed & fully sponsored by AstraZeneca UK Ltd. AstraZeneca UK Ltd is the manufacturer of the Turbu+ app whose registered address is 1 Francis Crick Avenue, Cambridge Biomedical Campus, Cambridge, CB2 0AA. United Kingdom, who is represented in your country by AstraZeneca Australia Pty Ltd with offices at 66 Talavera Rd, Macquarie Park, NSW, 2113 Australia.
The Turbu+ device has been designed and developed by Adherium Europe Ltd.
Approval ID: AU-2374/Prep Mar 2017/Next Update Due Mar 2018
AppAdvice does not own this application and only provides images and links contained in the iTunes Search API, to help our users find the best apps to download. If you are the developer of this app and would like your information removed, please send a request to takedown@appadvice.com and your information will be removed.