Electrochemistry is a tool for parameterization of oxidation-reduction reactions and electrochemical cells
Electrochemistry


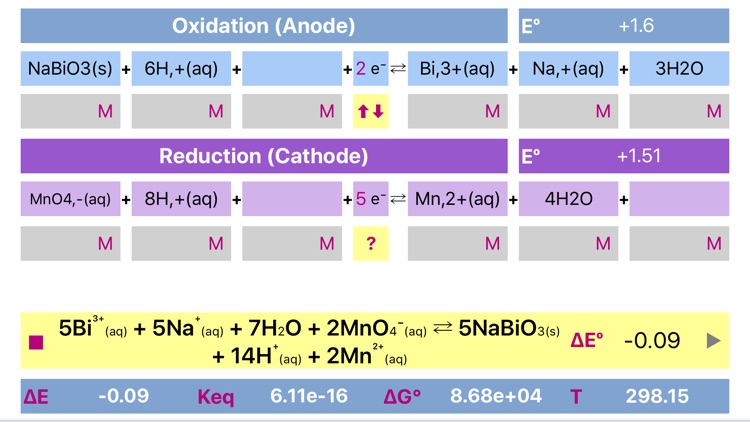
What is it about?
Electrochemistry is a tool for parameterization of oxidation-reduction reactions and electrochemical cells. The app helps to balance and combine a standard half-reactions into a single redox equation and to find the overall standard potential, equilibrium constant, standard free Gibbs energy and total voltage. The app includes redox reactions database, that automatically suggests and inserts reactions upon typing.

App Screenshots





App Store Description
Electrochemistry is a tool for parameterization of oxidation-reduction reactions and electrochemical cells. The app helps to balance and combine a standard half-reactions into a single redox equation and to find the overall standard potential, equilibrium constant, standard free Gibbs energy and total voltage. The app includes redox reactions database, that automatically suggests and inserts reactions upon typing.
Application Features:
The calculation results are valid either for electrochemical cell or a single reaction solution.
Initially, oxidation and reduction half-reactions are loaded into Anode (Oxidation) and Cathode (Reduction) parts respectively. The division can be done arbitrary, and may be easily changed (reversed) any time.
The application includes redox reactions database. Upon typing into the leftmost field of any of a half reactions, list of the suggested equations will appear. It is preferable to start typing from the less common element to get a more specific suggestions. The suggestions are case sensitive!
Both half reactions must be pre equilibrated stoichiometrically, as actually, they appear in standard tables. Importantly, same half-reaction may be equilibrated differently as a function of environment acidity.
Application helps to find whether cell is galvanic and spontaneous or electrolytic.
Voltage and potentials units are Volts only, Energy units are Joules, Temperature is in Kelvin only. Concentration units are adjusted by tapping the respective fields. If the compound is gas, then units should be set to "atm" and value should be adjusted appropriately. Solids and water are usually not taken into consideration for calculation of equilibrium constant, therefore they "concentration" fields must be left empty.
The process can be easily reversed by changing oxidation and reduction reactions by pressing yellow button placed between anode and cathode parts.
Long press on final reaction field copies equation to a clipboard for further usage.
Compounds and their charges should be separated by comma for proper representation.
The app is based on Nernst equation.
Temperature value (in Kelvin) can be changed to meet custom conditions.
AppAdvice does not own this application and only provides images and links contained in the iTunes Search API, to help our users find the best apps to download. If you are the developer of this app and would like your information removed, please send a request to takedown@appadvice.com and your information will be removed.