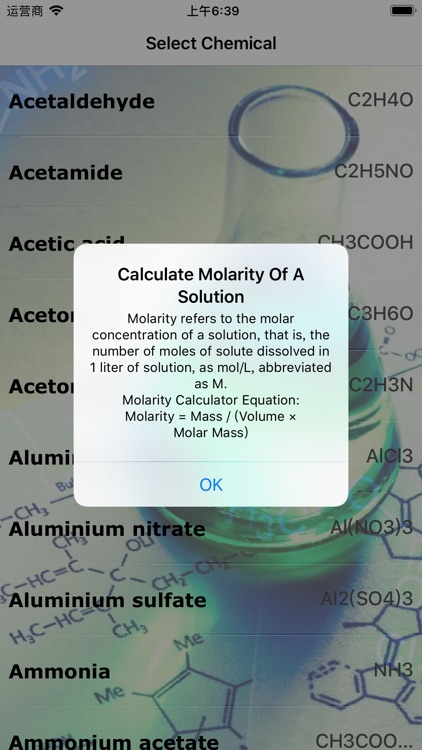
Molarity refers to the molar concentration of a solution, that is, the number of moles of solute dissolved in 1 liter of solution, as mol/L, abbreviated as M

Molarity Of Solution

What is it about?
Molarity refers to the molar concentration of a solution, that is, the number of moles of solute dissolved in 1 liter of solution, as mol/L, abbreviated as M. Now This app can help you to calculate molarity of Solution.Just Select Chemical and add Mass And Volume you can get molarity of your solution.This app is very valuable asset for chemists.We offer this app to students for more in depth knowledge of chemistry.

App Screenshots



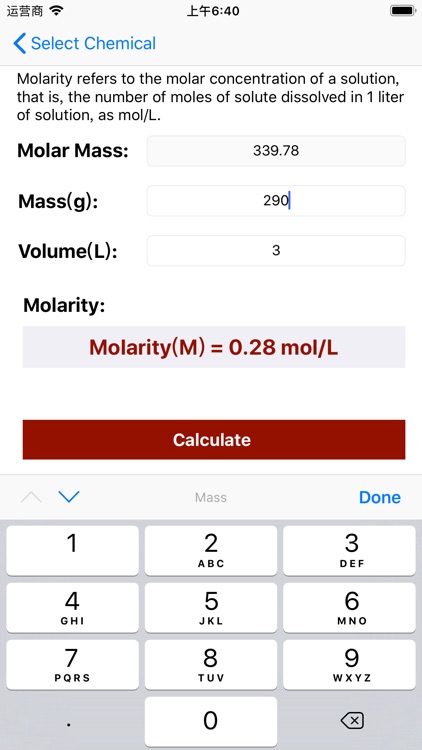
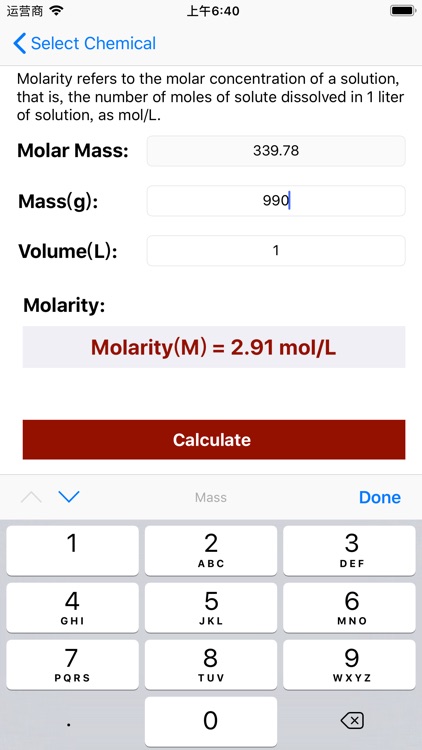
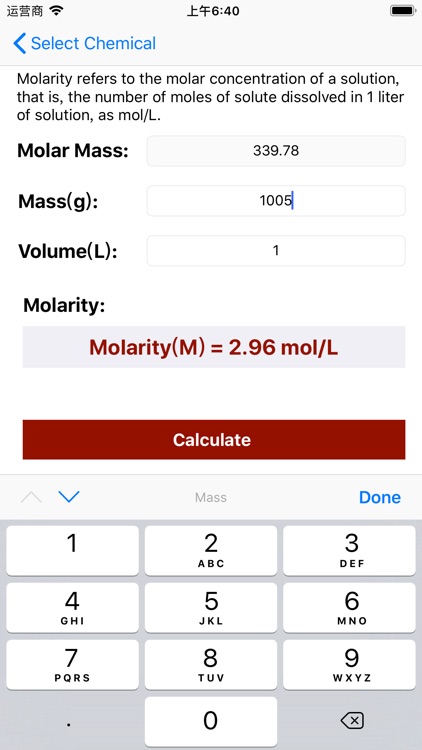
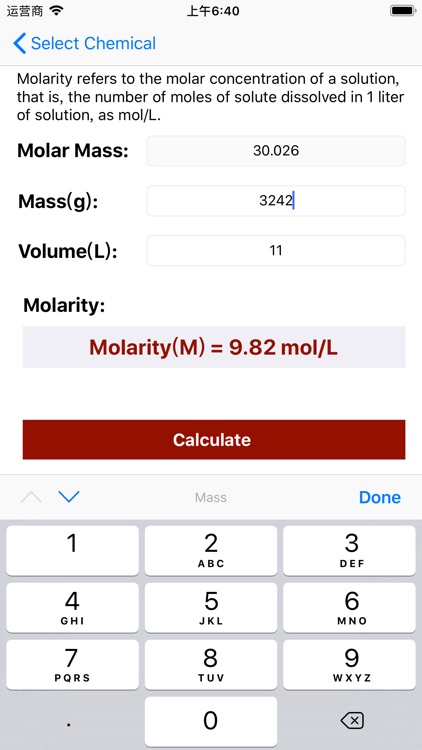
App Store Description
Molarity refers to the molar concentration of a solution, that is, the number of moles of solute dissolved in 1 liter of solution, as mol/L, abbreviated as M. Now This app can help you to calculate molarity of Solution.Just Select Chemical and add Mass And Volume you can get molarity of your solution.This app is very valuable asset for chemists.We offer this app to students for more in depth knowledge of chemistry.
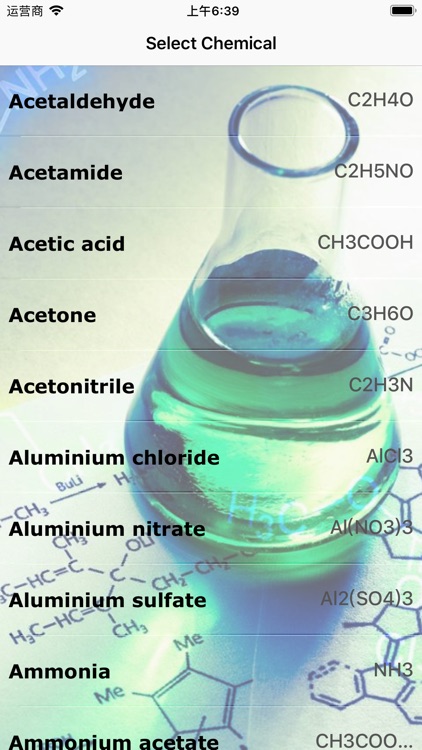
This app is specially used for quick calculation for solution and adjusting the concentration of liquid.We have collected many Chemicals for using its molar mass as best of our needs
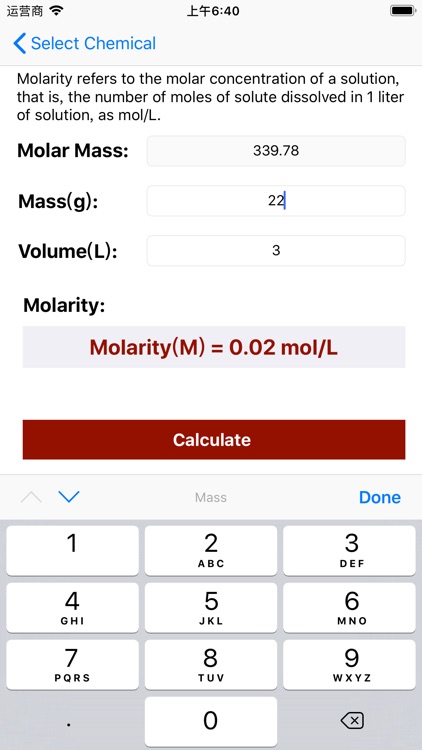
Molarity Calculator Equation:
Molarity = Mass / (Volume × Molar Mass);
Mole = Concentration (g/L) × Volume (L) / MW
Molarity Calculation Example:
Calculate moles of a 15ml NaCl solution with concentration 0.2g/L
The molar mass of NaCl is 58.44276 g/mol (Click here for molar mass calculator). So:
mole = 0.2 * (15 / 1000) / 58.44276 = 0.000051332278 (mol)
AppAdvice does not own this application and only provides images and links contained in the iTunes Search API, to help our users find the best apps to download. If you are the developer of this app and would like your information removed, please send a request to takedown@appadvice.com and your information will be removed.