
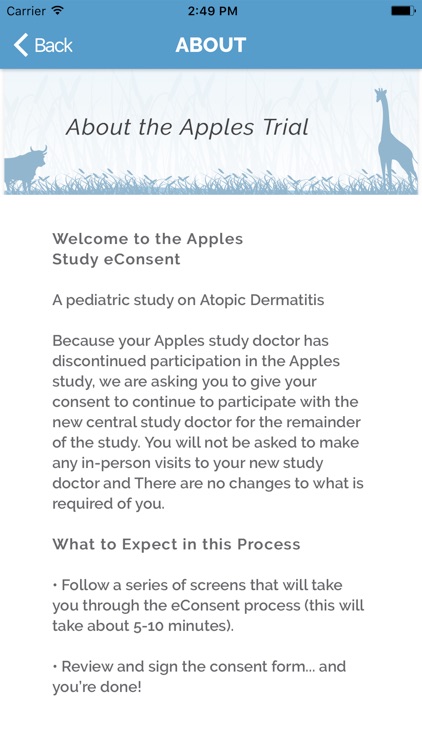
The My APPLES Study eConsent App is for already enrolled patients to provide their consent to continue to participate in the My APPLES Study with a new central study doctor

My APPLES Study


What is it about?
The My APPLES Study eConsent App is for already enrolled patients to provide their consent to continue to participate in the My APPLES Study with a new central study doctor.

App Store Description
The My APPLES Study eConsent App is for already enrolled patients to provide their consent to continue to participate in the My APPLES Study with a new central study doctor.
APPLES is a post-marketing (Phase IV), prospective, observational pediatric study assessing the long-term safety of tacromlimus ointment 0.03% or 0.1% (Protopic Ointment 0.03% or 0.1%) in the treatment of atopic dermatitis.
Through their mobile device, the My Apples Study App will conveniently allow patients to review the Informed Consent Form and provide their eSignature, consenting to participate in the remainder of the study.
AppAdvice does not own this application and only provides images and links contained in the iTunes Search API, to help our users find the best apps to download. If you are the developer of this app and would like your information removed, please send a request to takedown@appadvice.com and your information will be removed.