
You are invited to participate in an observational clinical study

NailClin
What is it about?
You are invited to participate in an observational clinical study.

App Screenshots



App Store Description
You are invited to participate in an observational clinical study.
This study investigates the safety, user experience and efficacy of Nailclin in an observational Post Market Clinical Follow-up, with a maximum duration of one year. We are in compliance with the applicable and local regulatory laws in Europe and New Zealand.
Nailclin is an approved medical device, limiting the risks, while you can track the progress of you nail. We recommend users to seek a doctor’s advice upfront or in addition to using this app and before making any medical decisions.
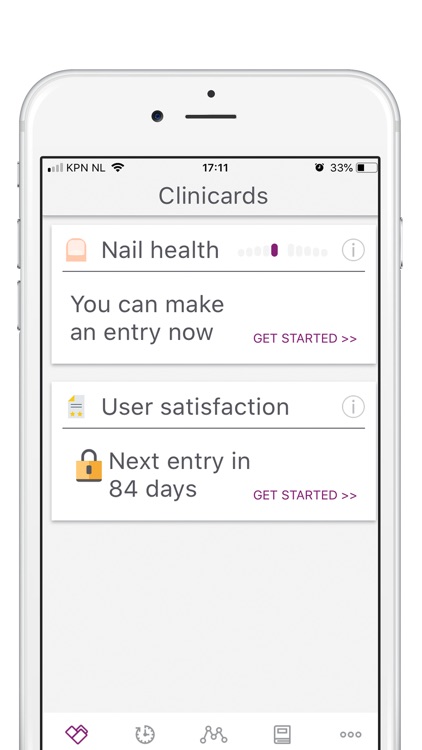
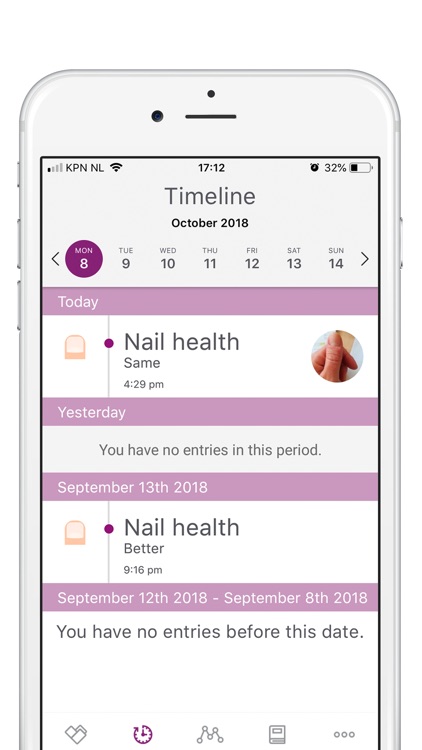
Participation will involve a time commitment. You will be asked to take photos (weekly for the first 8 weeks, and monthly thereafter) and fill in short questionnaires (baseline, week 8, month 3, 6, 9 and 12).
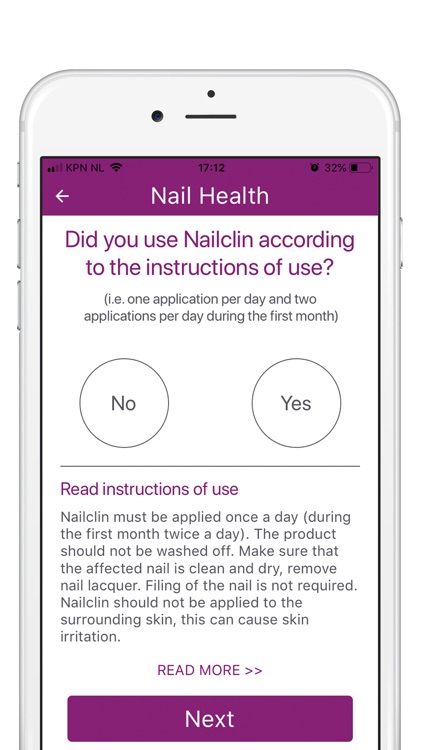
Your participation in this study will involve using the product Nailclin as instructed in the instruction for use leaflet (included in the packaging or available here (in app link)): twice daily during the first month and once daily as long as the nail is affected (up to 12 months).
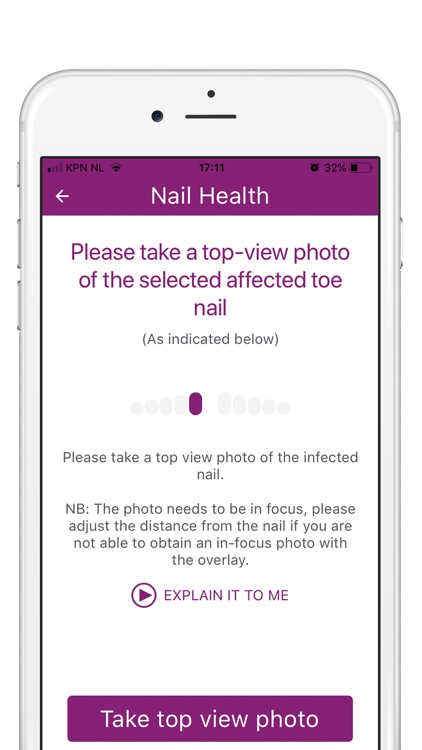
During the study, we’ll ask you to take photos to monitor the efficacy and safety of Nailcin. At each moment of observation two photos are asked to be taken; one frontal and one from above. Observations will take place once per week during the first 8 weeks and once monthly during the remainder of the study (maximum of one year).
A short questionnaire or survey (max 5 questions) will be conducted intermittently to assess user experience and to indicate side effects. There are 6 preset points during which the survey questions will be asked: at baseline when the app is installed, after 8 weeks to assess initial progress, at intervals of 3 months (3, 6 and 9 months) to assess overall progress, and finally, when you withdraw from the study or when the study comes to an end, whichever comes first.
AppAdvice does not own this application and only provides images and links contained in the iTunes Search API, to help our users find the best apps to download. If you are the developer of this app and would like your information removed, please send a request to takedown@appadvice.com and your information will be removed.