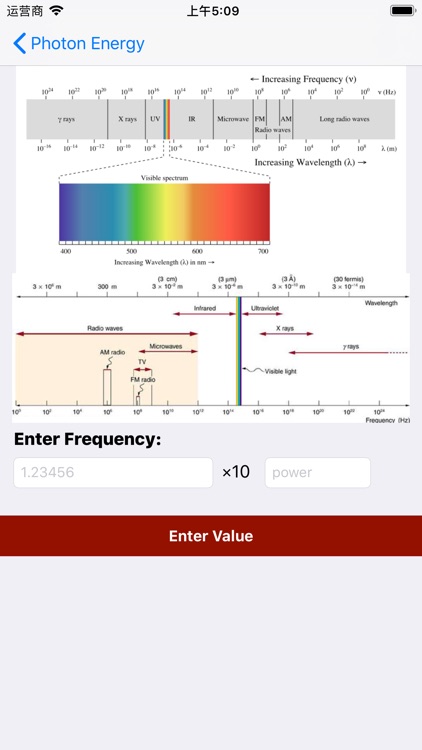
Now you can easily calculate the energy of photons by applied value of frequency
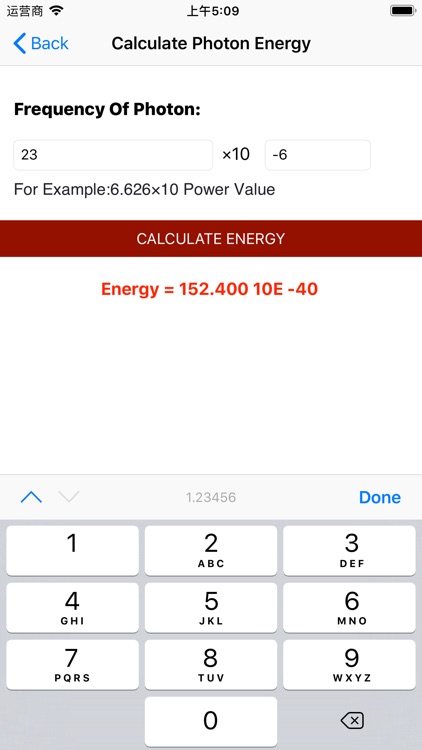
Calculate Photon Energy

What is it about?
Now you can easily calculate the energy of photons by applied value of frequency.As Energy of photon dependent on frequency of electromagnetic wave.This app helps us to learn and get a in-depth knowledge of Electromagnetic waves.This app is basically a a utility for researchers to calyulate energy in souls.As Energy is one the most important parameter for analyzing electromagnetic waves.

App Screenshots





App Store Description
Now you can easily calculate the energy of photons by applied value of frequency.As Energy of photon dependent on frequency of electromagnetic wave.This app helps us to learn and get a in-depth knowledge of Electromagnetic waves.This app is basically a a utility for researchers to calyulate energy in souls.As Energy is one the most important parameter for analyzing electromagnetic waves.
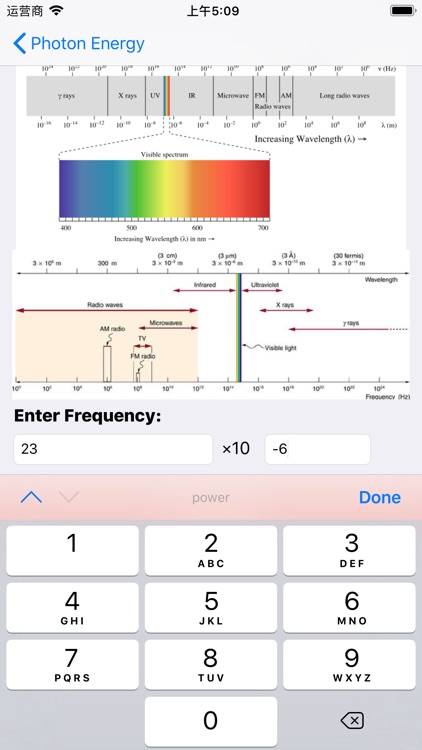
Planck’s discoveries paved the way for the discovery of the photon. A photon is the elementary particle, or quantum, of light. As we will soon see, photons can be absorbed or emitted by atoms and molecules. When a photon is absorbed, its energy is transferred to that atom or molecule. Because energy is quantized, the photon’s entire energy is transferred (remember that we cannot transfer fractions of quanta, which are the smallest possible individual “energy packets”). The reverse of this process is also true. When an atom or molecule loses energy, it emits a photon that carries an energy exactly equal to the loss in energy of the atom or molecule. This change in energy is directly proportional to the frequency of photon emitted or absorbed. This relationship is given by Planck’s famous equation:
E=hν
E, equals, h, where
E is the energy of the photon absorbed or emitted (given in Joules,J start text, J, end text),
ν is frequency of the photon (given in Hertz,
Hz
h is Planck’s constant,6.626×10−34 Js.
AppAdvice does not own this application and only provides images and links contained in the iTunes Search API, to help our users find the best apps to download. If you are the developer of this app and would like your information removed, please send a request to takedown@appadvice.com and your information will be removed.